On Tuesday, 29 October 2024, we hosted the first edition of our Enterprise AI Breakfast Series at our London office. This series of events is aimed at bringing together key members of the tech community to discuss the opportunities and challenges of AI in the enterprise. Our first event focused on AI in Healthcare and featured a panel of experts: Bijoy Sagar Executive VP and Chief Information Technology and Digital Transformation Officer at Bayer and Wim Van Hecke, co-founder and CEO at icometrix, and was moderated by our Founder & Managing Partner, Frederic Wohlwend.
Key Takeaways:
1. We are at an inflection point for AI in healthcare: in a post Attention is All You Need world, AI is “table stakes” for healthcare.
The integration of Large Language Models (LLMs) in healthcare presents significant opportunities, particularly given the exponential growth of electronic health records (EHRs), medical literature, and patient-generated data. These tools could significantly enhance healthcare professionals’ ability to extract insights and make informed decisions.
However, the complexity of healthcare data extends beyond traditional text and numerical formats, encompassing extensive imagery from radiology and non-standard data structures like ECG chart results. In Europe, initial adoption was somewhat constrained by the regulatory environment and relatively limited collaborative efforts, though research has continued steadily. The NHS in the UK has made progress in this area, granting closed access to certain developers for model training and developing the NHS-LLM.
A significant development occurred in April 2024 when Hugging Face, in partnership with Open Life Science AI and the University of Edinburgh, launched the Open Medical-LLM Leaderboard. This platform tracks, ranks, and evaluates LLMs’ performance in answering medical questions based on established medical datasets.
The MONAI framework represents another important initiative in this space. Originally established by NVIDIA and King’s College London, this open-source collaborative framework has expanded to include multiple collaborators, accelerating both research and clinical collaboration in medical imaging.
Several notable models have emerged as leaders in this field, including Google’s MedLM (formerly Med-PaLM 2), GPT-4 Vision, and Google PaLI 3.
2. AI may finally help fulfil the “promise” of personalised medicine
Traditionally, medical treatments have been designed for the “average” patient, often overlooking the unique genetic, biological, and environmental factors that contribute to an individual’s health and treatment response. However, the emerging field of personalised medicine holds the promise of “revolutionising” healthcare by tailoring interventions to the specific characteristics of each patient. Also known as precision medicine, this approach aims to maximise the effectiveness of treatments and minimise side effects by accounting for factors like genetics, biomarkers, and environmental influences that shape disease development and treatment outcomes. The vision of personalised medicine is to prescribe “the right treatment to the right patient in the right dose at the right time and expect an optimal outcome,” moving away from the one-size-fits-all approach of the past and towards a more individualised, targeted, and effective model of healthcare.
The transition to personalised medicine is one fraught with challenges both in the laboratory and in the clinical setting. While oncology has seen the most progress in precision medicine, with the approval of targeted therapies for specific genetic mutations, the broader adoption of personalised approaches remains slow. Some key barriers to widespread adoption of personalised medicine include:
Clinical Barriers:
• The high costs associated with genomic testing and companion diagnostics.
• The lack of expertise among healthcare professionals to interpret test results.
• Poorly integrated EHR (Electronic Health Record) systems with a lack of data and privacy standardisation.
Pharma / Drug Development Barriers:
• Difficulties in recruiting and enrolling the right patient populations for clinical trials of personalized medicines.
• Challenges in providing sufficient evidence of safety and efficacy for regulatory approval.
• High costs of incorporating new processes into innovative trial designs and manufacturing personalised treatments.
3. There is significant potential for AI in drug discovery but compute remains the main limiting factor
3.1 Pharma’s AI Moment
Recent advancements in AI combined with more widespread and affordable genetic testing have the potential to significantly impact and improve the drug development process and in doing so help overcome some of the Pharma barriers to widespread precision medicine.
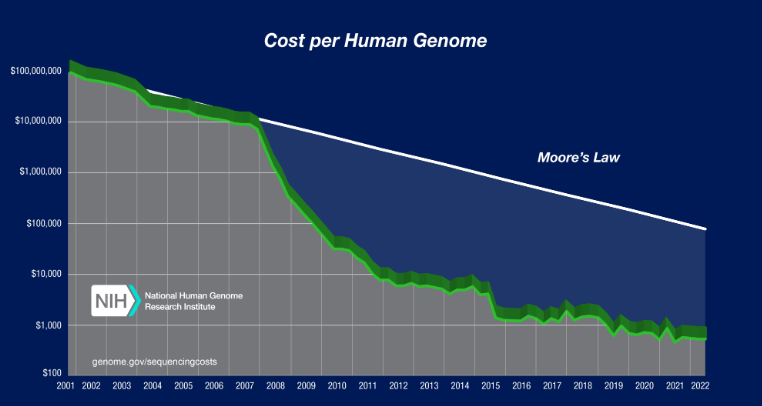
The advent of new sequencing technologies and increased competition between sequencing companies has led to a sharp decrease in the cost of sequencing a human genome, outpacing Moore’s Law (see graph below). This access to a new “treasure trove” of data holds enormous opportunity in drug discovery.
3.2 The Discovery phase at a glance
The discovery phase is about identifying or “discovering” a specific molecule – for example a DNA sequence, RNA molecule, or protein – that plays a critical role in a disease state and that can be targeted by a drug. This is called the target molecule.
Once the target molecule is identified, researchers search for a compound(s) that interact with it – and has the potential to become a drug candidate. Usually many are identified as candidates and researchers conduct a further series of experiments to on each to gauge their performance as a viable drug substance.
It generally takes 12–15 years from the initiation of a discovery programme to the point at which national drug‑regulatory agencies grant marketing approval. Source
According to a paper published by BCG & The Wellcome Trust AI could yield between 25%-50% time and cost saving in drug discovery up to the preclinical stage. If we assume that:
• The cost of bringing a drug to market is $1Bn on average.
• That 60% of those costs can be attributed to the pre-clinical stage ($600M).
• That 50% of pre-clinical stage costs is R&D related ($300M).
• And finally that AI could yield up to a 50% cost-saving in this stage.
We can estimate that there is up to $150M of potential ROI for AI solutions, per new drug, at the pre-clinical R&D stage alone.
3.3 AI use-cases in Drug Discovery
Research Applications:
• Target Identification: ML models trained on protein sequences or structures can predict properties (e.g. efficacy, safety) and design optimized proteins. AI can also explore broader chemical space to identify novel, previously “undruggable” targets. A leading example is AlphaFold.
• Lead Compound Discovery: AI predicts how well drug molecules bind to targets and assesses preclinical data to fast-track promising candidates.
• De Novo Design: New AI models can design entirely novel drug molecules, rather than just matching existing compounds to known targets (example)
Lab Tools & Automation: AI and lab automation are accelerating in-vitro research.
• Virtual Screening: AI can screen large libraries of compounds in silico to narrow down those most likely to bind to targets—reducing experimental workload and improving compound selection.
• Robotic Workstations: Robots automate lab tasks like sample prep and analysis, improving speed, accuracy, and reproducibility. These workflows also generate more data to train better AI models.
4. AI is still a raw technology with significant barriers to adoption in the clinical setting
4.1 Adoption and Readiness
Bringing AI into healthcare—especially in areas involving complex data systems—can take 3–4 years from procurement to productive use. Adoption is often slowed by operational, regulatory, and technical hurdles.
4.2 Operational Challenges: Silos and Collaboration Gaps
AI development in healthcare often happens in isolation. Researchers may lack access to real patient data, and procurement processes mirror these silos. Implementation also requires a rare mix of technical and clinical expertise—data engineers to manage extraction and integration, and clinicians to ensure meaningful data interpretation. Unlike traditional translational medicine, which bridges science and clinical practice, medical AI demands close collaboration across three groups: data scientists, engineers, and clinicians.
Although partnerships between healthcare systems and academia are common, formal agreements for secure, multi-site data sharing remain rare. To avoid delays, collaboration frameworks should be established early—ideally through “communities of practice” that bring together teams across disciplines and institutions to share solutions or push for unified agreements.
4.3 Regulatory Challenges
AI in medicine operates in a heavily regulated space, where data protection, safety, and ethical standards are paramount. Generative AI raises added concerns about explainability and “black box” decisions. GDPR remains a major consideration due to the personal nature of healthcare data.
A breakthrough came in 2023 when Huma became the first company to receive EU MDR Class IIb certification for a disease-agnostic Software-as-a-Medical-Device (SaMD). Huma’s platform supports remote monitoring, clinical assessments, and patient self-management tools. Its regulatory readiness sets a precedent, lowering the barrier to market for others and accelerating digital health innovation.
4.4 Technical Challenges: Interoperability
Healthcare has long struggled with poor data sharing and system compatibility. The FHIR (Fast Healthcare Interoperability Resources) standard, developed by HL7, offers a modern, flexible framework for exchanging electronic health records (EHRs). Major EHR vendors like Cerner and Epic have adopted FHIR, creating standardized profiles to guide integration.
FHIR has become a key part of healthcare data infrastructure, used widely across providers and pharma companies. While adoption varies globally—faster in the U.S. than in Europe—it’s increasingly foundational to health tech development.
A huge thank you to our panellists from everyone on the Forestay team. If you are a founder building in the healthcare AI space, we’d love to hear from you. You can contact us via Forestay’s website or email at contact@forestay.vc. Please stay tuned for upcoming events and more thought-provoking discussion.
